DHEA: Evidence-Based Support for Postmenopausal Sexual Health
Our Team
5/15/2025
Dehydroepiandrosterone (DHEA), a hormone precursor to androgens and estrogens, naturally declines in postmenopausal women, contributing to sexual dysfunction, such as reduced libido and vaginal dryness, affecting up to 50% of this population. FERTINATAL® DHEA for Women , a dietary supplement de livering 25 mg of pharmaceutical-grade DHEA per capsule, is designed to restore hormonal balance and potentially enhance sexual function. Clinical studies suggest improvements in sexual desire, arousal, and vaginal health, though results are somewhat inconsistent.
We here offer scientific evidence supporting DHEA’s role in improving sexual activity among postmenopausal women and provide practical guidance for its use. Our focus is on FERTINATAL® DHEA because it is in its micronization size and, therefore, absorption rate, the DHEA used in the original DHEA studies performed at The Center for Human Reproduction (CHR) in New York City which introduced DHEA use into female infertility treatments worldwide. Most other DHEA products on the market deviate in characteristics and, therefore, may not offer the same results.
These CHR conducted studies also resulted in several U.S. patents for use of androgens, including DHEA, in infertile women with low androgen levels, the only such patents awarded in the U.S., and held by the producer of FERTINATAL® DHEA, OVATERRA. It is formulated with only five clean ingredients, ensuring purity and safety and is available for purchase via at the website of OVATERRA (myovaterra.com), - a subsidiary of Fertility Nutraceutical, LLC, and it is also available on Amazon. Offers of FERTINATAL® at other secondary markets – which have recently appeared for the first time - should be viewed with caution and may not contain the real product.
Product Overview
FERTINATAL® DHEA for Women is a patented dietary supplement containing 25 mg of pharmaceutical grade dehydroepiandrosterone (DHEA) per capsule. Initially designed to support hormonal balance and reproductive function in properly selected infertile women, it has also attracted attention as a potential treatment to improve sexual arousal, vaginal health, and overall well-being in perimenopausal and postmenopausal women. In treating infertile women with DHEA, the CHR’s investigators, indeed, noticed that, especially perimenopausal women, spontaneously reported improved sexual function. They consequently performed a formal questionnaire study which confirmed this impression (Kushnir et al., 2019). A U.S. patent application making this claim is pending.
Scientific Background
DHEA is an androgen (male) hormone with very low affinity to the androgen receptor on cells. It, in itself, at low dosages, therefore, demonstrates very limited androgen hormonal activity in humans. It’s potential importance in female infertility, therefore, does not lie in its minimal direct androgen effects. Instead, it works through the principal male hormone, testosterone, because DHEA is the precursor from which the different organs in our body produce testosterone. Because organs take only as much DHEA out of circulation as they need to produce testosterone to the level the organ requires (and those levels vary between organs), to overdose on androgen activity with DHEA supplementation is rare. This stands in contrast to direct testosterone administration (usually given by transdermal gel), where overdosing is very common. And too high testosterone levels may be even more harmful to a woman’s fertility than too low levels. The CHR, therefore, from the beginning preferred DHEA supplementation over direct testosterone supplementation.
Sexual dysfunction, including reduced libido and vaginal atrophy, affect many women starting in perimenopause and affects up to 50% of postmenopausal women, significantly impacting quality of life. Declining androgen levels contribute to these symptoms. As already noted above, DHEA, produced in roughly similar proportions by the adrenal glands and ovarian theca cells, serves as a precursor to testosterone and, to a lesser degree estrogens (e.g., female hormones). Especially in postmenopausal women, peripheral conversion of DHEA to testosterone and estrogen becomes a critical source of sex steroids.
Key Clinical Evidence
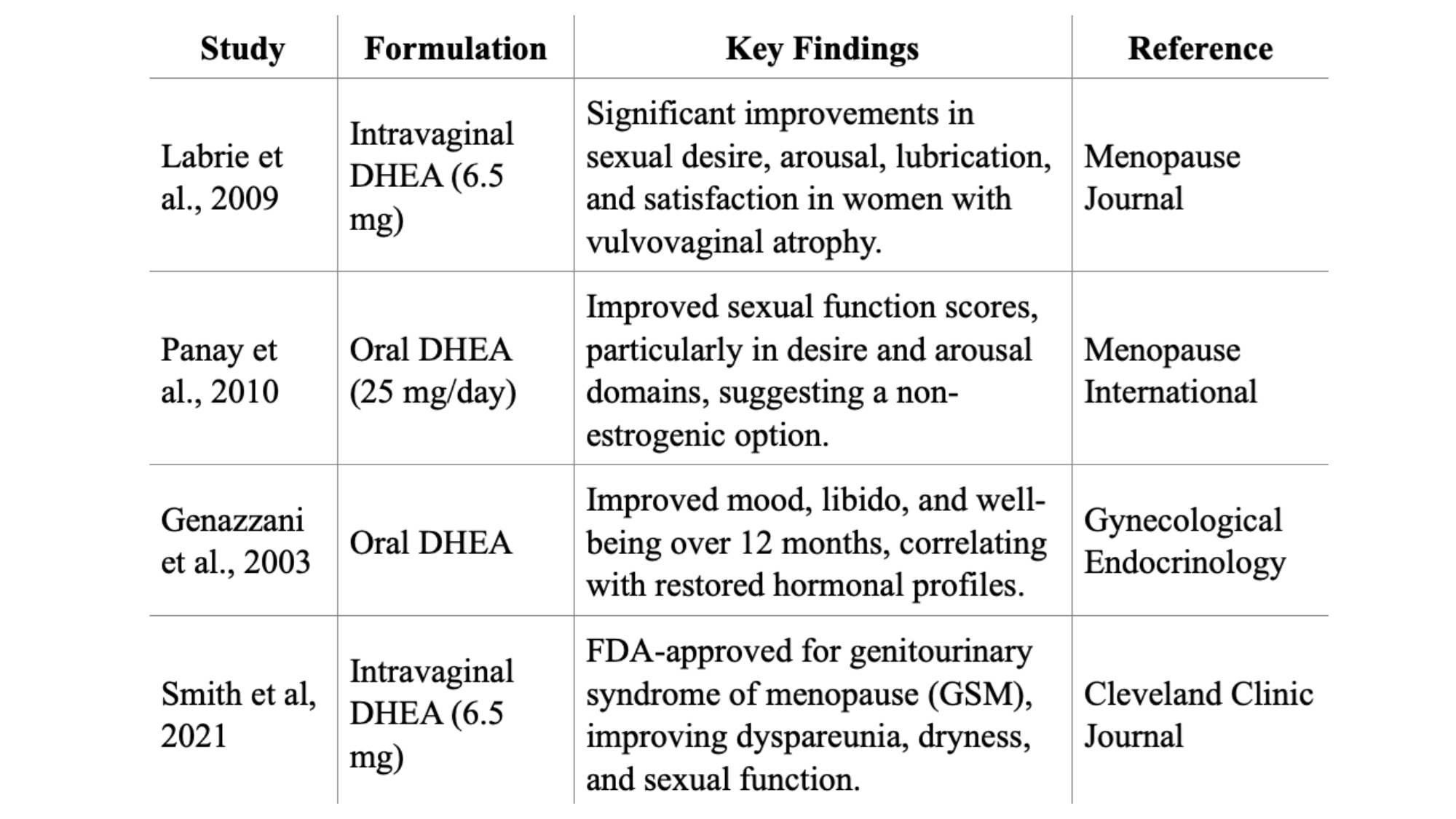
The efficacy of DHEA supplementation in postmenopausal women has been extensively studied, with both oral and intravaginal formulations showing promise for specific indications. Here are the studies in the literature on this subject, except for previously noted CHR study (Kushnir, et al., 2019). As will be obvious, the number of studies in the literature is unfortunately very small.
Intravaginal DHEA (Prasterone): This product is the only DHEA product FDA-approved for any postmenopausal indication in women, - in this case moderate to severe dyspareunia in the genitourinary syndrome of menopause (GSM) – under the claims that DHEA improves vaginal dryness, irritation, and sexual function domains, with neutral endometrial effects after 12 months. Clinical trials by Labrie et al. showed that daily administration of 6.5 mg intravaginal DHEA significantly improved dyspareunia, vaginal dryness, and other symptoms of vulvovaginal atrophy compared to placebo. The authors also reported improvements in vaginal pH, epithelial integrity, and sexual function domains, with minimal adverse effects, primarily limited to vaginal discharge (Labrie et al., 2016; Labrie et al., 2018).
Oral DHEA: Studies suggest improvements in sexual function and mood, but results are inconsistent. A 2011 review found that placebo-controlled RCTs do not consistently support oral DHEA for sexual function, well-being, or metabolic parameters (Davis et al., 2011). Previously noted CHR study, however, reported that following supplementation with DHEA, all serum androgen levels increased (each P < 0.0001), while FSH levels decreased by 2.6 ± 4.4 from a baseline of 10.3 ± 5.4 mIU/mL (P = 0.009). The Female Sexual Function Index (FSFI) score for the whole study group increased by 7% (from 27.2 ± 6.9 to 29.2 ± 5.6; P = 0.0166). Domain scores for desire increased by 17% (P = 0.0004) and by 12% for arousal (P = 0.0122); lubrication demonstrated an 8% trend towards improvement (P = 0.0551), while no changes in domain scores for orgasm, satisfaction, or pain were observed. Women in the lowest starting FSFI score quartile (<25.7), experienced a 6.1 ± 8.0 (34%) increase in total FSFI score following DHEA supplementation. Among these women, improvements in domain categories were noted for desire (40%), arousal (46%), lubrication (33%), orgasm (54%), satisfaction (24%), and pain (25%).
Bone Health: DHEA also improves bone mineral density in postmenopausal women with low bone mass (BM), potentially aiding osteoporosis prevention. A pooled analysis of four clinical trials indicated that DHEA therapy led to increases in lumbar spine BMD and trochanter BMD in women, while maintaining total hip BMD (Jankowski et al., 2006; Jankowski et al., 2019; Lin et al., 2019; Wierman et al 2014; Quester et al., 2022)
&srotate=0)
Clinical Applications
For gynecologists and endocrinologists, FERTINATAL® DHEA offers:
- An only minimally estrogenic, over-the-counter option for patients seeking alternatives to hormone replacement therapy (HRT).
- A clinically effective dosage (25 to 75 mg daily), aligning with studies showing benefits for sexual function.
- Easy purchase access via the OVATERRA website (myovaterra.com) as well as Amazon, facilitating patient compliance.
- A strong safety profile with minimal side effects.
Mechanism of Action
Peri- and postmenopausal orally administered DHEA is primarily converted in peripheral tissues to androgens (e.g., testosterone, androstenedione) and estrogens (e.g., estrone, estradiol), exerting localized as well as systemic effects, including:
- Improved vaginal lubrication and tissue elasticity.
- Enhanced sexual desire and responsiveness.
- Potential improvements in mood, quality of life, and bone health.
(Tang et al., 2021; Rutkowski et al., 2014; Klinge et al., 2018
Safety and Considerations
- Dehydroepiandrosterone (DHEA) is generally well-tolerated with no serious adverse effects reported in clinical trials. Minor side effects include oily skin, acne, mild hirsutism, hair loss.
- Intravaginal DHEA minimally affects systemic hormone levels, making it safe even for breast cancer survivors.
- Caution should be advised for patients with hormone-sensitive cancers due to DHEA's potential conversion to estrogen.
- Clinicians should monitor patients for hormonal changes or symptoms given still limited long-term safety data.
(Wierman et al., 2014; Barton et al., 2018; Barton et al., 2018; Labrie et al., 2008)
How long does it take to feel changes
The time it takes to feel changes with dehydroepiandrosterone (DHEA) treatment in peri- and postmenopausal women can vary. Studies have reported effects within a few weeks to several months. This stands in contrast to DHEA utilization in infertile women, where ovarian effects are seen as early as 6-8 weeks after supplementation start. Caufriez et al. reported that a 3-week administration of 50 mg DHEA daily resulted in increased levels of testosterone and estradiol, with individual clinical responses varying widely (Caufriez et al., 2013). Another study by Genazzani et al., (2003) indicated that hormonal changes and improvements in climacteric symptoms were observed within 3 to 6 months of low-dose (25 mg/day) DHEA supplementation (Genazzani et al., 2003).
Product Access and Composition
- Product Name: FERTINATAL® DHEA For Women
- Active Ingredient: Dehydroepiandrosterone (DHEA), 25 mg per capsule
- Form: Oral capsules
- Classification: Dietary supplement (USA)
- Availability: Direct purchase at OVATERRA website (myovatera.com) and via Amazon (FERTINATAL® on Amazon)
Conclusions
FERTINATAL® DHEA offers an evidence-based approach to improving sexual arousal, vaginal health, and well-being in postmenopausal women. Its FDA-approved intravaginal counterpart for GSM, combined with its non-estrogenic profile and clean formulation, makes it an attractive option for clinicians. While oral DHEA’s benefits are less conclusive, its potential for GSM, sexual dysfunction, and bone health, supported by guidelines, warrants consideration. Clinicians should discuss benefits and risks with patients, emphasizing its role in targeted symptoms when other treatments are unsuitable.
REFERENCES
• Barton DL, Shuster LT, Dockter T, Atherton PJ, Sloan JA, Sood R, Loprinzi CL. Systemic and local effects of vaginal dehydroepiandrosterone (DHEA): NCCTG N10C1 (Alliance). Support Care Cancer. 2018 Apr;26(4):1335-1343. doi:10.1007/s00520-017-3960-9. PMID: 29164377
• Barton DL, Sloan JA, Shuster LT, Gill P, Griffin P, Flynn K, Terstriep SA, Rana FN, Dockter T, Atherton PJ, Tsai M, Sturtz K, Lafky JM, Riepl M, Thielen J, Loprinzi CL. Evaluating the efficacy of vaginal dehydroepiandrosterone for vaginal symptoms in postmenopausal cancer survivors: NCCTG N10C1 (Alliance). Support Care Cancer. 2018 Feb;26(2):643-650. doi: 10.1007/s00520-017-3878-2. Epub 2017 Sep 18. PMID: 28921241; PMCID: PMC5754227
• Caufriez A, Leproult R, L'Hermite-Balériaux M, Kerkhofs M, Copinschi G. Effects of a 3-week dehydroepiandrosterone administration on sleep, sex steroids and multiple 24-h hormonal profiles in postmenopausal women: a pilot study. Clin Endocrinol (Oxf). 2013 Nov;79(5):716-724. doi:10.1111/cen.12201.
• Davis SR, Panjari M, Stanczyk FZ. Clinical review: DHEA replacement for postmenopausal women. J Clin Endocrinol Metab. 2011 Jun;96(6):1642-1653. doi:10.1210/jc.2010-2888. PMID: 21411558.
• Genazzani AD, Stomati M, Bernardi F, Pieri M, Rovati L, Genazzani AR. Long-term low-dose dehydroepiandrosterone oral supplementation in early and late postmenopausal women modulates endocrine parameters and synthesis of neuroactive steroids. Fertil Steril. 2003 Dec;80(6):1495-1501. doi: 10.1016/j.fertnstert.2003.06.005. PMID: 14667889.
• Jankowski CM, Gozansky WS, Schwartz RS, Dahl DJ, Kittelson JM, Scott SM, Van Pelt RE, Kohrt WM. Effects of dehydroepiandrosterone replacement therapy on bone mineral density in older adults: a randomized, controlled trial. J Clin Endocrinol Metab. 2006 Aug;91(8):2986-2993. doi: 10.1210/jc.2005-2484. Epub 2006 May 30. PMID: 16735495.
• Jankowski CM, Wolfe P, Schmiege SJ, Nair KS, Khosla S, Jensen M, von Muhlen D, Laughlin GA, Kritz-Silverstein D, Bergstrom J, Bettencourt R, Weiss EP, Villareal DT, Kohrt WM. Sex-specific effects of dehydroepiandrosterone (DHEA) on bone mineral density and body composition: A pooled analysis of four clinical trials. Clin Endocrinol (Oxf). 2019 Feb;90(2):293-300. doi: 10.1111/cen.13901. Epub 2018 Dec 9. PMID: 30421439; PMCID: PMC6336516.
• Klinge CM, Clark BJ, Prough RA. Dehydroepiandrosterone research: past, current, and future. Vitam Horm. 2018;108:1-28. doi: 10.1016/bs.vh.2018.02.002. Epub 2018 Mar 16. PMID: 30029723.
• Kushnir VA, Darmon SK, Barad DH, Weghofer A, Gleicher N. Effects of dehydroepiandrosterone (DHEA) supplementation on sexual function in premenopausal infertile women. Endocrine. 2019 Mar;63(3):632-638. doi: 10.1007/s12020-018-1781-3. Epub 2018 Oct 11. PMID: 30311171.
• Labrie F, Archer D, Bouchard C, Fortier M, Cusan L, Gomez JL, Girard G, Baron M, Ayotte N, Moreau M, Dubé R, Côté I, Labrie C, Lavoie L, Berger L, Gilbert L, Martel C, Balser J. Effect of intravaginal dehydroepiandrosterone (Prasterone) on libido and sexual dysfunction in postmenopausal women. Menopause. 2009 Sep-Oct;16(5):923-931. doi: 10.1097/gme.0b013e31819e85c6. PMID: 19424093.
• Labrie F, Archer DF, Koltun W, Vachon A, Young D, Frenette L, Portman D, Montesino M, Côté I, Parent J, Lavoie L, Beauregard A, Martel C, Vaillancourt M, Balser J, Moyneur É; VVA Prasterone Research Group. Efficacy of intravaginal dehydroepiandrosterone (DHEA) on moderate to severe dyspareunia and vaginal dryness, symptoms of vulvovaginal atrophy, and of the genitourinary syndrome of menopause. Menopause. 2018 Nov;25(11):1339-1353. doi: 10.1097/GME.0000000000001238. PMID: 30358731.
• Labrie F, Cusan L, Gomez JL, Côté I, Bérubé R, Bélanger P, Martel C, Labrie C. Effect of intravaginal DHEA on serum DHEA and eleven of its metabolites in postmenopausal women. J Steroid Biochem Mol Biol. 2008 Sep;111(3-5):178-194. doi: 10.1016/j.jsbmb.2008.06.003. Epub 2008 Jun 12. PMID: 18598765.
• Lin H, Li L, Wang Q, Wang Y, Wang J, Long X. A systematic review and meta-analysis of randomized placebo-controlled trials of DHEA supplementation of bone mineral density in healthy adults. Gynecol Endocrinol. 2019 Nov;35(11):924-931. doi: 10.1080/09513590.2019.1616175. Epub 2019 Jun 25. PMID: 31237150.
• Panay N, Al-Azzawi F, Bouchard C, Davis SR, Eden J, Lodhi I, Rees M, Rodenberg CA, Rymer J, Schwenkhagen A, Sturdee DW. Testosterone treatment of HSDD in naturally menopausal women: the ADORE study. Climacteric. 2010 Apr;13(2):121-131. doi: 10.3109/13697131003675922. PMID: 20166859.
• Quester J, Nethander M, Eriksson A, Ohlsson C. Endogenous DHEAS is causally linked with lumbar spine bone mineral density and forearm fractures in women. J Clin Endocrinol Metab. 2022 Apr 19;107(5):e2080-e2086. doi: 10.1210/clinem/dgab915. PMID: 34935937; PMCID: PMC9016453.
• Rutkowski K, Sowa P, Rutkowska-Talipska J, Kuryliszyn-Moskal A, Rutkowski R. Dehydroepiandrosterone (DHEA): hypes and hopes. Drugs. 2014 Jul;74(11):1195-1207. doi: 10.1007/s40265-014-0259-8. PMID: 25022952.
• Smith T, Batur P. Prescribing testosterone and DHEA: the role of androgens in women. Cleve Clin J Med. 2021 Jan 1;88(1):35-43. doi: 10.3949/ccjm.88a.20030. PMID: 33384313.
• Tang J, Chen LR, Chen KH. The utilization of dehydroepiandrosterone as a sexual hormone precursor in premenopausal and postmenopausal women: an overview. Pharmaceuticals (Basel). 2021 Dec 29;15(1):46. doi: 10.3390/ph15010046. PMID: 35056103; PMCID: PMC8781653.
• Wierman ME, Arlt W, Basson R, Davis SR, Miller KK, Murad MH, Rosner W, Santoro N. Androgen therapy in women: a reappraisal: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2014 Oct;99(10):3489-3510. doi: 10.1210/jc.2014-2260. PMID: 25279570.
Recent Posts
Mental Health and IVF: Navigating The Terrain No One Warned You About
Mental Health and IVF: Navigating The Terrain No One Warned You About
Understanding Ovulation: A Brief Summary to Optimize Your Fertility
Learn how hormones, lifestyle, and tracking tools can optimize ovulation and boost your chances of conception.
The Impacts of Stress on Fertility: Optimizing Your Fertility
Practical ways to manage the stress that naturally arises during any fertility treatment.